HYDROGEN VENTILATION USING HIGH AND LOW VENTS
A battery enclosure using high and low vents is shown in Figure 2A & 2B. This type of ventilation system takes advantage of the natural property of light-density gas to rise upward, and heavier-density gas to sink downward. Under most common conditions, Hydrogen has a lighter density than air and tends to rise upward when in contact with air. Warmer air is less dense than cooler air, and so warm air tends to push upwards when in contact with cooler air.
The ventilation system shown in Example 2 is driven more by air temperature differences than by hydrogen concentration, and can therefore cause unwanted temperature extremes to occur in the battery box by providing too much fresh air when it is not needed.
Hydrogen Ventilation Using High and Low Vents. Cold Day Ventilation by Convection.
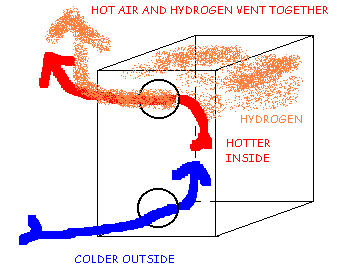 Figure 2A shows that on a cool day, the lighter warm air and lighter Hydrogen will rise together out the high vent, drawing fresh cool air in through the low vent. Both the temperature of the warm air and the presence of hydrogen will drive the ventilation rate. Increases in either the temperature or the concentration of hydrogen inside the box will increase the flow of fresh air. Under these conditions, this vent system may work quite well.
Figure 2A shows that on a cool day, the lighter warm air and lighter Hydrogen will rise together out the high vent, drawing fresh cool air in through the low vent. Both the temperature of the warm air and the presence of hydrogen will drive the ventilation rate. Increases in either the temperature or the concentration of hydrogen inside the box will increase the flow of fresh air. Under these conditions, this vent system may work quite well.
FIGURE 2B
Hydrogen Ventilation Using High and Low Vents, Warm Day Hydrogen Trap.
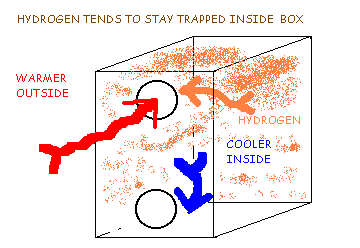
Figure 2B show that on a warm day, when the inside temperature is cooler than outside, the direction of air flowing through the vents can reverse. When this happens, warm air trying to enter the top vent pushes back the Hydrogen trying to rise out the same vent, causing the hydrogen to stagnate and collect inside the box. The box becomes a hydrogen trap and diffusion soon mixes the hydrogen with the air throughout the box. Under the right conditions, this will cause explosive levels to be reached inside the box.
